1. Introduction
Trisomy 8 is one of the most frequent cytogenetic abnormalities in acute myeloid leukemia (AML). Trisomy 8 frequently occurs with additional aberrant or complex karyotypes; it can also occur as the sole cytogenetic abnormality. While prior studies have shown that trisomy 8 cooperates with other somatic genetic mutations (e.g., RUNX1), there is persistent controversy about the prognostic impact of AML with trisomy 8. We analyzed the relationship between trisomy 8 and additional molecular lesions, the ELN 2022 revision, and the prognostic impact of trisomy 8 across modern treatment strategies.
2. Methods
We analyzed 627 patients from the Project ERIS database with newly diagnosed AML treated from January 1, 2013 to April 18, 2023 at VCU Massey Comprehensive Cancer Center. We identified 86 patients with trisomy 8 independent of the presence of complex cytogenetics and a cohort of 541 historical controls without trisomy 8. We recorded baseline patient-related and disease characteristics, including age, ECOG, Charlson comorbidity index (CCI) scores, molecular profiling and ELN 2022 cytogenetic risk, dates of regimen initiation, and survival. We used the D'Agostino & Pearson method for normality testing and the t-test or Mann-Whitney (as applicable) tests for between-group comparisons. Categorical comparisons used Fischer's exact test. We applied the Bonferroni correction if multiple comparisons were made. The Wilson-Brown method was used for 95% confidence intervals. We analyzed survival by the Kaplan-Meier method with significance determined by the log-rank test. The event for calculating the overall survival (OS) was the date of death. Patients were otherwise censored at the date of last contact.
3. Results
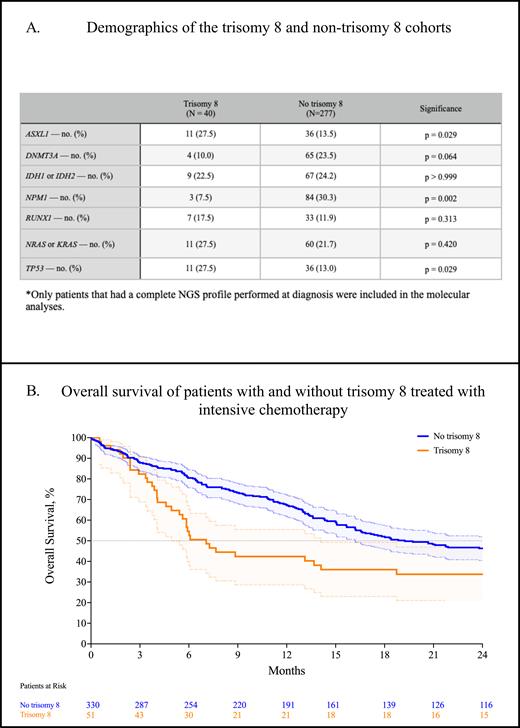
The most common cytogenetic abnormalities associated with trisomy 8 were the presence of a complex karyotype (45.2%, 95% CI: 35.0-55.9), loss of 5 (15.5%, 95% CI, 9.3-24.7%), loss of 7 (8.3%, 95% CI, 4.1-16.2), and -17p (13.1%, 95% CI, 7.5-21.9). ASXL1 mutations tended to occur significantly more frequently in trisomy 8 compared to those without (27.5% vs 13.0%, p = 0.029, Table A). Similarly, TP53 mutations occurred significantly more frequently in trisomy 8 than those without (27.5% vs 13.0%, p = 0.029). Conversely, DNMT3A mutations were less likely to be associated with trisomy 8, which approached statistical significance (10.0% vs 23.47%, p = 0.063). NPM1 mutations were significantly less likely to be associated with trisomy 8 compared to those without (7.5% vs 30.3%, p = 0.002). Consequently, trisomy 8 was significantly more likely to be associated with ELN 2022 adverse risk disease (odds ratio: 2.754, p < 0.0001).
Next, we compared response and survival with intensive chemotherapy using conventional 7+3 in those with and without trisomy 8. In 51 patients with trisomy 8, the composite complete response rate (CRc; CR + CRi + CRh) was 34.1% (95% CI, 21.9-48.9), significantly worse compared to 330 patients without trisomy 8 (55.1%, 95% CI, 49.4-60.6, p = 0.010). The median overall survival was significantly worse in patients with trisomy 8 treated with 7+3 compared to those without trisomy 8 (7.1 m. vs 19.2 m., p = 0.009, Figure B). In 13 patients treated with venetoclax and a hypomethylating agent (HMA), the CRc was 25.0% (95% CI, 8.9-53.2), which was nonsignificantly worse compared with 87 patients without trisomy 8 that demonstrated a CRc of 48.4% (95% CI, 36.4-60.6, p = 0.206). The overall survival of patients treated with venetoclax + HMA nonsignificantly favored patients without trisomy 8 (8.2 m. vs 5.0 m., p = 0.268).
4. Discussion
AML with trisomy 8 is significantly enriched in ASXL1 and TP53 mutations; conversely, trisomy 8 is significantly less likely to harbor NPM1 mutations. Patients with trisomy 8 are significantly more likely to have ELN 2022 adverse-risk disease. The CRc rate and median overall survival are significantly worse in patients with trisomy 8 treated with intensive chemotherapy. We observed no significant difference in response or survival in patients with or without trisomy 8 treated with venetoclax + HMA.
Disclosures
Grant:Prescient Therapeutics: Research Funding. Maher:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Sobi (Doptelet): Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal